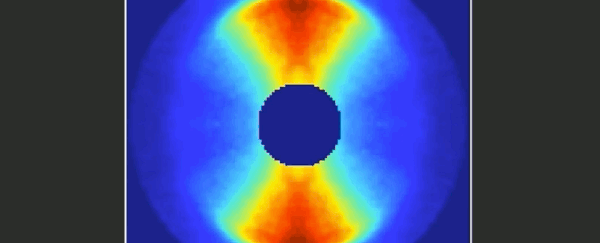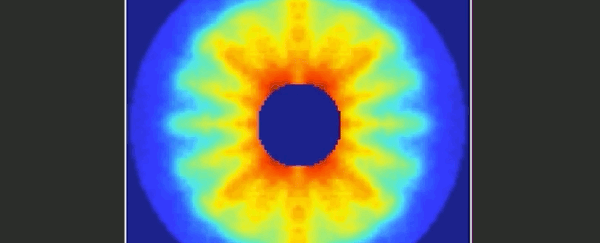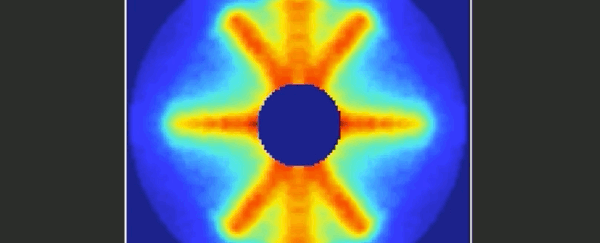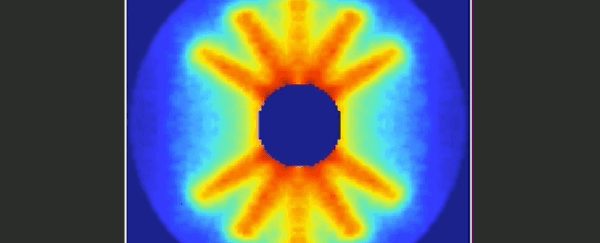
Imagine trying to film an event that was over and done within a mere 125 trillionths of a second. It's something that molecular physicists have long been dreaming of, and at last it seems they've achieved their goal.
Using precisely tuned pulses of laser light, an international team of scientists from four different institutions has managed to film the ultrafast rotation of a molecule.
"We recorded a high-resolution molecular movie of the ultrafast rotation of carbonyl sulphide as a pilot project," said molecular physicist Evangelos Karamatskos from DESY, Germany's largest accelerator centre.
"The level of detail we were able to achieve indicates that our method could be used to produce instructive films about the dynamics of other processes and molecules."
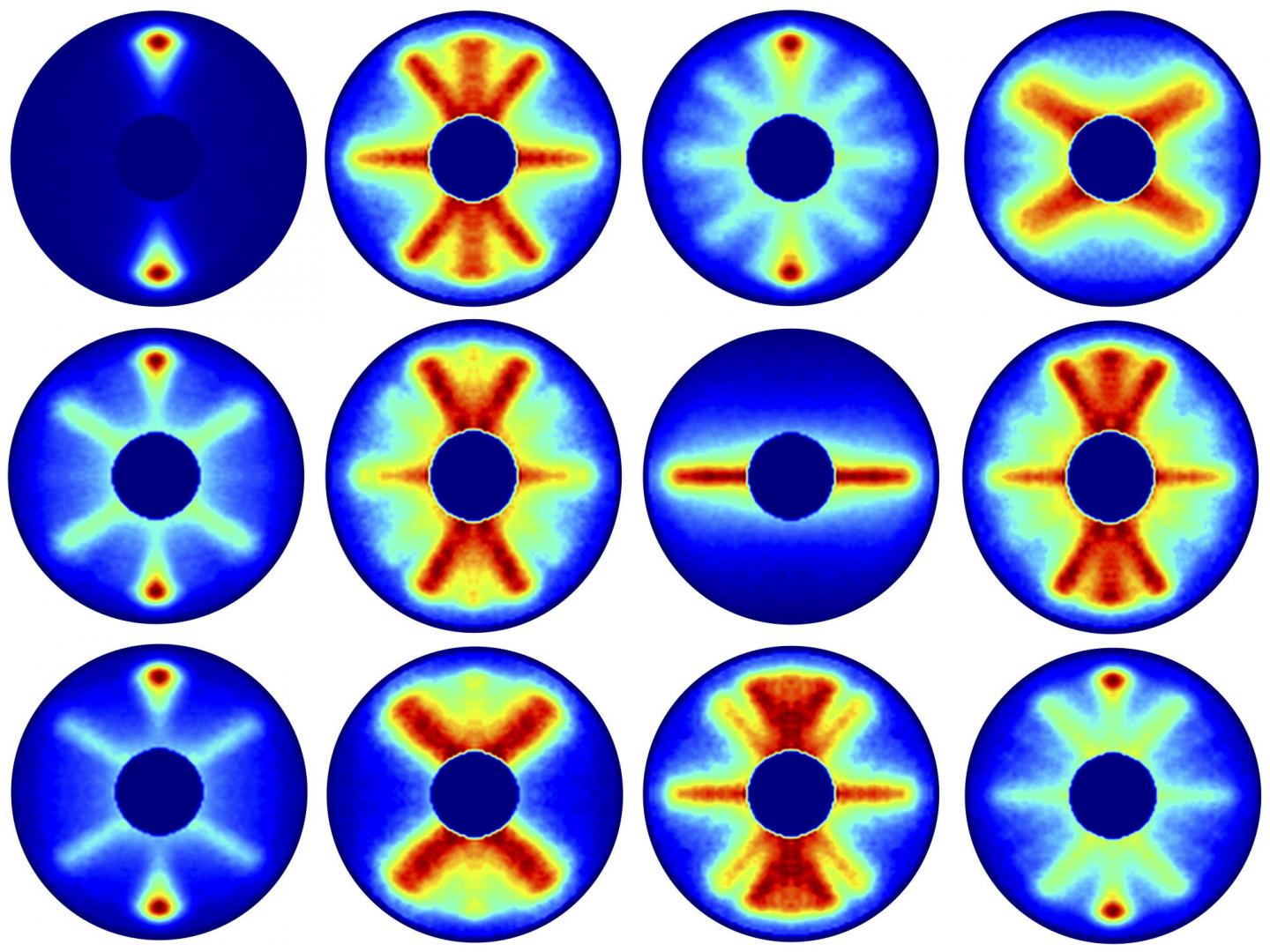
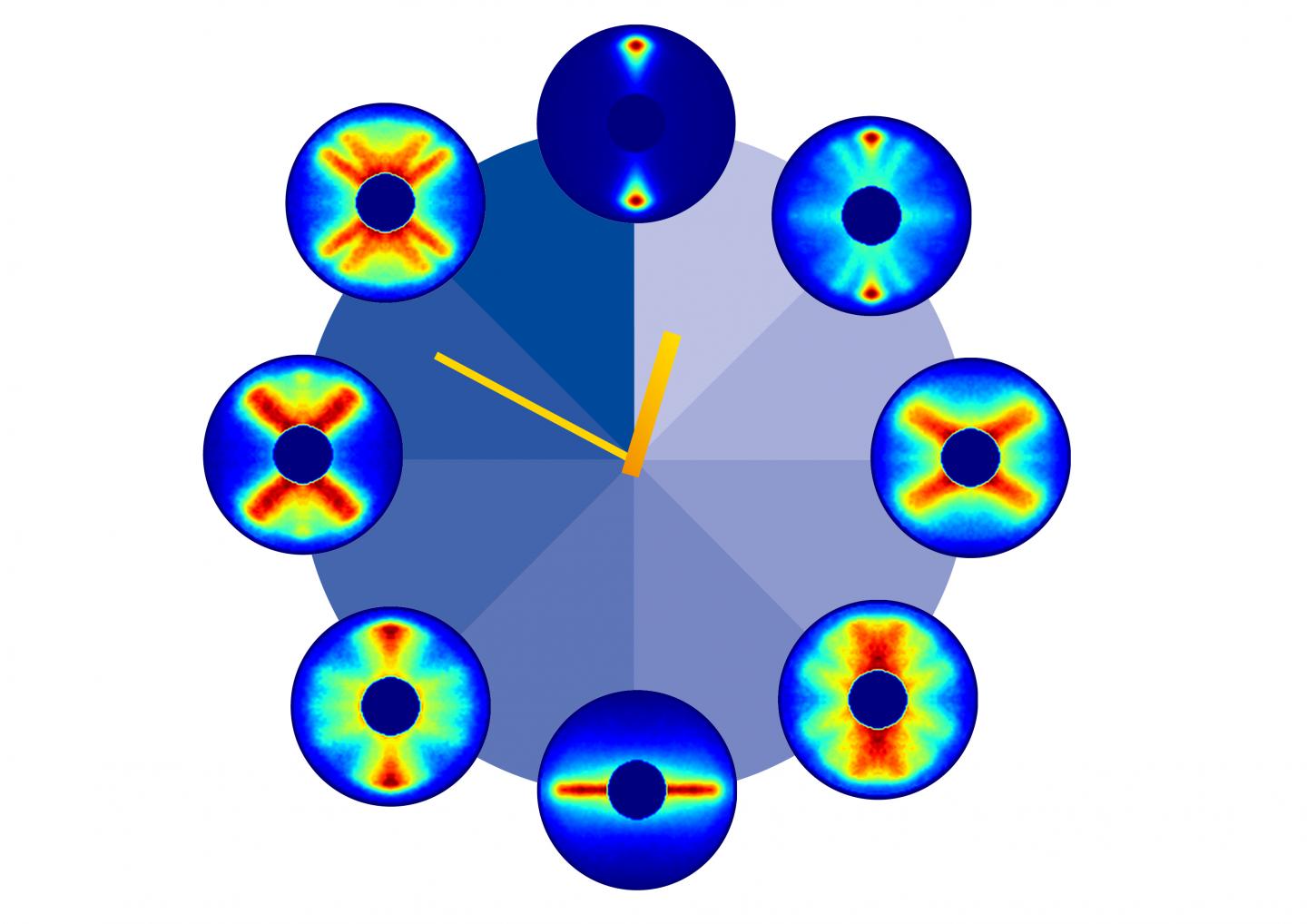
Together, the video below represents 651 pictures, assembled sequentially to cover one and a half rotations of the carbonyl sulphide molecule. The final product is a 125 picosecond film of the molecule, slowed way down for your enjoyment.
There's no denying its beauty, but the footage is even more magnificent when you understand what precisely you're looking at.
When a substance is in a gaseous state, the molecules are relatively far apart and therefore free to undergo rotation around their axes. This rotation is subject to the rules of quantum mechanics.
As a simple and common sulphur gas, the rod-shaped carbonyl sulphide - whose molecules consist of one oxygen, one carbon and one sulphur atom - is therefore the perfect twirling model.
But the head of the research team, physicist Jochen Küpper, says you shouldn't think of this molecule rotating like a stick.
"The processes we are observing here are governed by quantum mechanics. On this scale, very small objects like atoms and molecules behave differently from the everyday objects in our surroundings," explains Küpper, who works at the University of Hamburg and DESY.
"The position and momentum of a molecule cannot be determined simultaneously with the highest precision; you can only define a certain probability of finding the molecule in a specific place at a particular point in time."
Even when the molecule points in multiple directions at the same time, each of these has a different probability according to quantum mechanics.
"It is precisely those directions and probabilities that we imaged experimentally in this study," says molecular researcher Arnaud Rouzée from the Max Born Institute in Berlin.
"From the fact that these individual images start to repeat after about 82 picoseconds, we can deduce the period of rotation of a carbonyl sulphide molecule."
 (Evangelos Karamatskos/DESY)
(Evangelos Karamatskos/DESY)
To get the gas molecules moving in unison, the team first used two pulses of infrared laser light, precisely tuned to each other so that they pulsed every 38 trillionths of a second (picosecond).
The next step then included a further laser pulse with a longer wavelength, which was used to determine the position of the molecules at intervals of about 0.2 trillionths of a second.
The whole process was painstaking work, as this latter pulse destroys the molecules. Each snapshot, therefore, represents a whole new experiment starting over again.
 (Evangelos Karamatskos/DESY)
(Evangelos Karamatskos/DESY)
The authors hope that their new technique can help us study other molecules and processes, like the internal twisting that occurs in molecules or chiral compounds, which are compounds that exist in two forms, each a mirror image of the other.
"Furthermore, the very high degree of field-free alignment achieved here would be extremely useful for stereochemistry studies as well as for molecular-frame imaging experiments," the team concludes in their study.
The research has been published in Nature Communications.